Hybridization of Atomic Orbitals - Hybridization is the mixing of atomic orbitals in an atom to generate a set of new atomic orbitals, called hybrid orbitals. Hybrid orbitals, which are atomic orbitals obtained when two or more nonequivalent orbitals of the same atom combine, are used to form covalent bonds. Typical hybrid orbitals are sp3, sp2, sp, sp3d and sp3d2.
Here are some guidelines of hybridization:
The hybridization concept is applied to explain the bonding nature in a molecule, not an isolated atom.
Hybridization is the mixing of two or more nonequivalent atomic orbitals.
The number of hybrid orbitals via mixing is equal to the number of pure atomic orbitals that involve in the hybridization.
Covalent bonds in polyatomic molecules are formed by the overlap of hybrid orbitals, or hybrid orbitals with unhybridized ones.
This module is to calculate and display the MO energy diagram of diatomic molecules and show the hybrid orbitals of polyatomic molecules.
This is an one-step solution. In the case of diatomic molecules, the MO diagram with filled electron is shown, for polyatomic molecules (more than two atoms in a molecule), the 3D MO picture is displayed.
1. Click Hybrid/MO tab to open Hydrid/MO module.
On the left pane, it is the Compute module and one the right it is the Guide page. The top pane is the navigational toolbar.
2. Enter the formula of a molecule from Input field - Enter a Molecular Formula, in this case, NO3-.
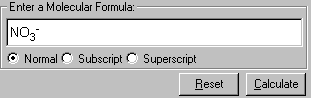
Note: To enter subscript and superscript, use the Subscript and Superscript options below the input box. Alternatively, you can use <Up Arrow> to enter superscript and <Down Arrow> to enter subscript. The keyboard sequence to enter NO3- is N-O-<Down Arrow>-3-<Up Arrow>-"-".
3. Click Calculate to proceed. If you need to clear the input field, click Reset.
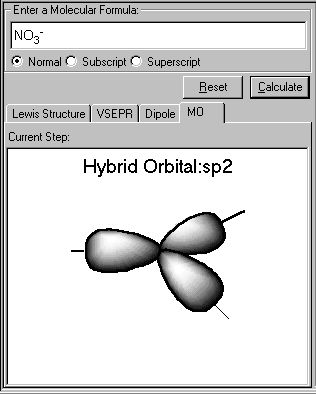
The sp3 hybrid orbitals of the central atom N is displayed. The three sp2 orbital lie in a plane and form a trigonal planar arrangement. Each of the N-O bond is formed by the overlap of a nitrogen sp2 hydride orbital and an oxygen 2p orbital. The NO3- molecule is planar and all ONO angles are 120°. This result conforms to VSEPR prediction.