VSEPR Theory- The valence-shell electronic-pair repulsion (VSEPR) model is an approach of using the number of electrons surrounding a central atom to study the molecular structures, based on the theory that the structure around a given atom is determined principally by minimizing electron-pair repulsions.
Here are some guidelines for applying the VSEPR model:
1. First, write the Lewis structure of the molecule, in this model, consider only the electron pairs of the central atom.
2. Count the total number of electron pairs around the central atom, including both the bonding pairs and lone pairs. Usually the multiple bonds are counted as single bonds, one pair per bond.
3. Use the VSEPR geometry table to predict the geometry of the molecule.
4. In predicting bond angles, the repulsion of the lone pair is stronger than that of the bonding pair.
This module is to predict the shape of a molecule in which the central atom is surrounding by the pairs of valance electrons. This is done by using the dot structure and deciding how many bonding electron pairs are involved.
This is an one-step solution. The 3D structure of a molecule and the type of the molecular geometry are displayed. The electron configuration of the central atom is shown in terms of total numbers of electron pairs, bonding pairs and non-bonding pairs.
1. Click VSEPR tab to open VSEPR module.
On the left pane, it is the Compute module and one the right it is the Guide page. The top pane is the navigational toolbar.
2. Enter the formula of a molecule from Input field - Enter a Molecular Formula, in this case, NO3-.
Note: To enter subscript and superscript, use the Subscript and Superscript options below the input box. Alternatively, you can use <Up Arrow> to enter superscript and <Down Arrow> to enter subscript. The keyboard sequence to enter NO3- is N-O-<Down Arrow>-3-<Up Arrow>-"-".
3. Click Calculate to proceed. If you need to clear the input field, click Reset.
The VSEPR screen appears. The VSEPR structure of the input molecule is displayed, along with the name of the geometrical structure. The total electron pairs, bonding pairs and non-bonding pairs are tabulated on the same screen. From these three set of numbers, the molecular geometry is determined.
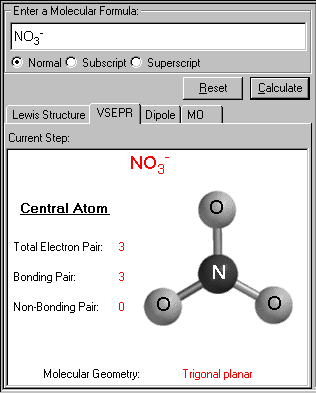
In the case of NO3-, the trigonal planar geometry is found. The central atom N has no lone pair(s) left, all three bonds count for one bonding pair each. Notice the double bond here counts for only one bonding pair, just like the single bond.
The guide is shown on the right to explaining the rules of VSEPR.