Half-Reaction Method (or Ion-Electron Method) is to split an unbalanced redox equation into two half-reactions, oxidation and reduction half-reactions. Both half-reactions are then balanced in according to the change of electrons. It is applied the best for redox reactions with ions and in aqueous solution.
Chemical equations with changes of oxidation numbers can be balanced using half-equation method. This module can guide you through step-by-step in balancing redox reactions using this method. Here are the steps:
Separate into half-reactions.
Balance the oxidizing and reducing elements in each half-reaction.
Balance atoms other than O and H.
In acidic solution, balance oxygen with H2O. In basic solution, balance oxygen with OH- and H2O.
In acidic solution, balance hydrogen with H+. In basic solution, balance hydrogen with OH- and H2O.
Balance each half-reaction for charge with e-.
Multiply each half-reaction by a coefficient so the numbers of electrons are the same in each and add both half-equations together.
Cancel common reactants and products.
Eliminate fractions by multiplying a common factor if needed.
Complete the equation by final inspection.
The Wizard for Half-Equation Method walks through step-by-step in balancing an equation with detailed guide side-by-side.
1. Open the Balance Equation module and enter the unbalanced equation.
Here is the typing instruction:
To enter normal text, just type in as is, be sure to use both cases for element symbols, e.g. Fe (Not FE). Be sure the Input Option - Normal is selected (default).
To enter the equation sign, you can use either "=" or "--->"or "<--->" symbols.
To enter superscripts for the charge species (Ca2+), there are two ways to input them:
Option 1: Select Superscript in the Input Option and type in ONE character, the option selection will return back to Normal. Click Superscript again, and enter the second superscript character. For example, the keystroke sequence to enter Ca2+ is C-a-"Superscript"-2-"Superscript"-"+".
Option 2: Press UpArrow key, the enter a superscript character, if needed, press UpArrow key again and then type in the second superscript character. For example, the keystroke sequence to enter Ca2+ is C-a-UpArrow-2-UpArrow-"+".
To enter subscripts for atom counters (H2), follow the similar instructions above, but use Subscript option box or DownArrow.
To enter a hydrated compound (CuSO4•5H2O), type in "." (period). The period (.) will be converted to the dot symbol(•) automatically upon typing in.

2. Upon entering the equation, click Balance Wizard to start the Equation Wizards. The Wizard selection screen appears. There are four wizards that you can select. In this case, click on Half-Reaction Method either in acidic or basic solution.

3. Click Next to proceed. The first screen of the Half-Equation wizard appears.
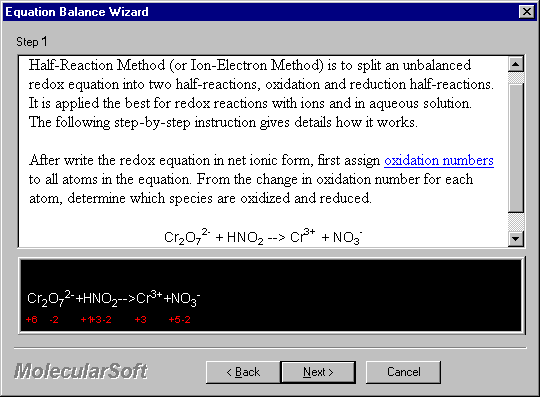
After write the redox equation in net ionic form, first assign oxidation numbers to all atoms in the equation. From the change in oxidation number for each atom, determine which species are oxidized and reduced.
4. Click Next. The next screen appears.
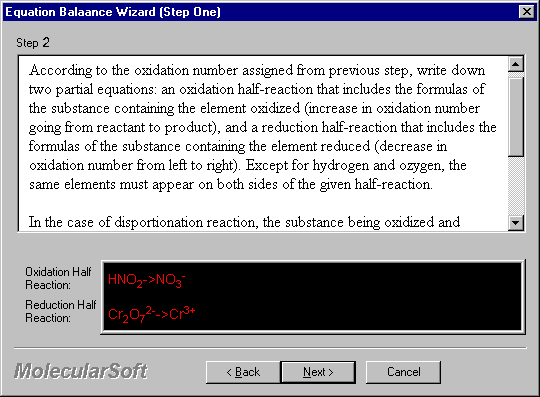
According to the oxidation number assigned from previous step, write down two partial equations: an oxidation half-reaction that includes the formulas of the substance containing the element oxidized (increase in oxidation number going from reactant to product), and a reduction half-reaction that includes the formulas of the substance containing the element reduced (decrease in oxidation number from left to right). Except for hydrogen and oxygen, the same elements must appear on both sides of the given half-reaction.
In the case of disproportionation reactions, the substance being oxidized and reduced simultaneously should be in both oxidation and reduction half-reactions.
5. Click Next.
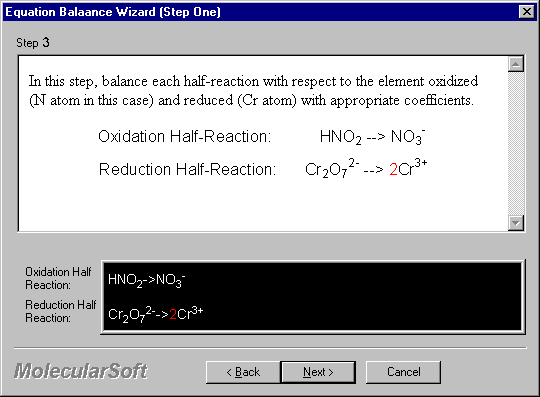
In this step, balance each half-reaction with respect to the element oxidized (N atom in this case) and reduced (Cr atom) with appropriate coefficients.
6. Click Next.
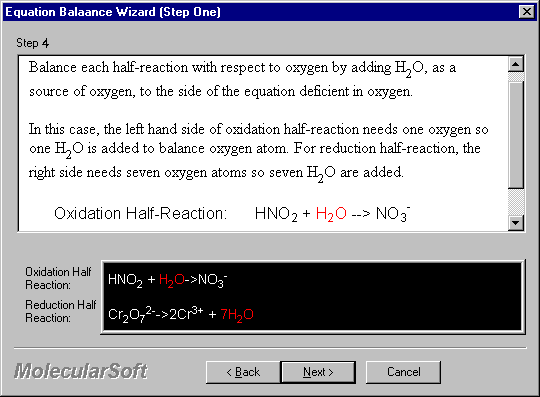
Balance each half-reaction with respect to oxygen by adding H2O, as a source of oxygen, to the side of the equation deficient in oxygen.
In this case, the left hand side of oxidation half-reaction needs one oxygen so one H2O is added to balance oxygen atom. For reduction half-reaction, the right side needs seven oxygen atoms so seven H2O are added.
7. Click Next.
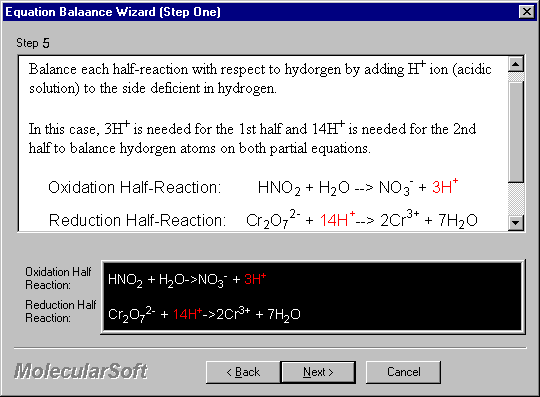
Balance each half-reaction with respect to hydrogen by adding H+ ion (acidic solution) to the side deficient in hydrogen.
In this case, 3H+ is needed for the 1st half and 14H+ is needed for the 2nd half to balance hydrogen atoms on both partial equations.
8. Click Next.

Balance each half-reaction with respect to total charge by adding electrons (e-) to the side with more positive total charge.
In the oxidation half-reaction below, total charge of left side is zero and that of right side is +2 (-1+3), so 2e- are needed for the right side. Similarly for the reduction half-reaction, 6e- are needed for the left side to balance the charge.
9. Click Next.
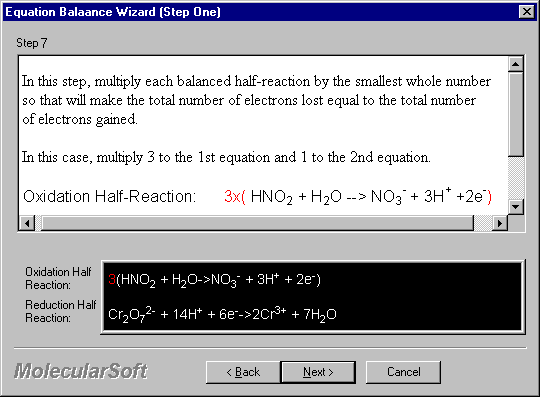
In this step, multiply each balanced half-reaction by the smallest whole number so that will make the total number of electrons lost equal to the total number of electrons gained.
In this case, multiply 3 to the 1st equation and 1 to the 2nd equation.
10. Click Next.
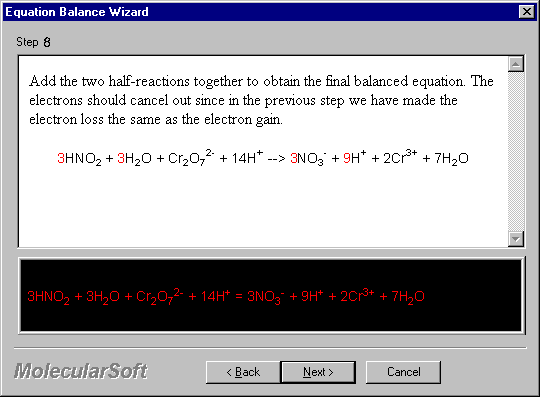
Add the two half-reactions together to obtain the final balanced equation. The electrons should cancel out since in the previous step we have made the electron loss the same as the electron gain.
11. Click Next.
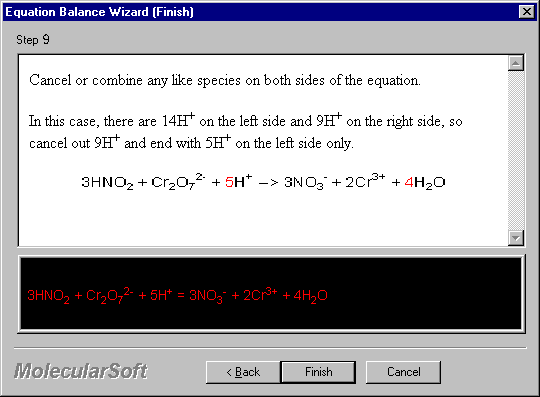
Cancel or combine any like species on both sides of the equation.
In this case, there are 14H+ on the left side and 9H+ on the right side, so cancel out 9H+ and end with 5H+ on the left side only.
12. Click Finish. The wizard walk-through is complete and it returns to the Equation Balance main screen with the balanced equation displayed in red.
