The basic idea of balancing equation by inspection is to strategically pick an element and balance it first, and then element-by-element to finish them. The basic principle is the law of conservation in mass - the mass of each atom before and after a reaction is unchanged. Other criteria can be used in balancing an equation include the conservation of oxidation numbers for redox reactions and conservation of charges for ionic reactions. The order of atoms to be balanced is important here. We will show you step-by-step how this method works by highlighting the working element and coefficients in red.
The balancing procedure is sometimes called "balancing by inspection". It is a trial and error method and is usually successful without any set rules. However, if you follow the guidelines below the process will be simpler.
1. Identify the most complicated formula, the formula having the largest number of atoms and/or the largest number of elements.
2. Balance the elements one at a time in the following order.
Start with the elements in the most complicated formula that are in only one other formula.
Save for last: elements appearing in more than two formulas uncombined elements
3. If fractions were used in step 2, clear them by multiplying all coefficients by the lowest common denominator.
4. Remove any "1" coefficients.
5. For more complicated equations, use the Advanced Inspection Methods.
5. Check the entire equation.
The Wizard for Inspection Method walks through step-by-step in balancing an equation with detailed guide side-by-side.
1. Open the Balance Equation module and enter the unbalanced equation.
Here is the typing instruction:
To enter normal text, just type in as is, be sure to use both cases for element symbols, e.g. Fe (Not FE). Be sure the Input Option - Normal is selected (default).
To enter the equation sign, you can use either "=" or "--->"or "<--->" symbols.
To enter superscripts for the charge species (Ca2+), there are two ways to input them:
Option 1: Select Superscript in the Input Option and type in ONE character, the option selection will return back to Normal. Click Superscript again, and enter the second superscript character. For example, the keystroke sequence to enter Ca2+ is C-a-"Superscript"-2-"Superscript"-"+".
Option 2: Press UpArrow key, the enter a superscript character, if needed, press UpArrow key again and then type in the second superscript character. For example, the keystroke sequence to enter Ca2+ is C-a-UpArrow-2-UpArrow-"+".
To enter subscripts for atom counters (H2), follow the similar instructions above, but use Subscript option box or DownArrow.
To enter a hydrated compound (CuSO4•5H2O), type in "." (period). The period (.) will be converted to the dot symbol(•) automatically upon typing in.
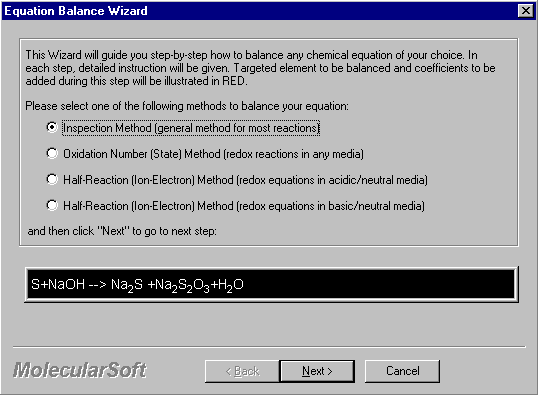
2. Click Balance Wizard to start the Equation Wizards. The Wizard selection screen appears. There are four wizards you can select. In this case, click on Inspection Method.
Click Next to proceed.
3. The first screen of Inspection Wizard appears.
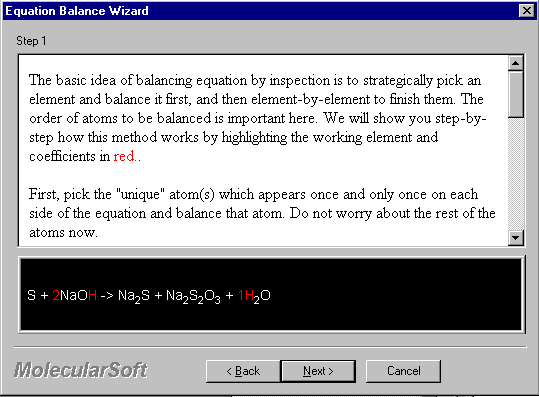
First, pick the "unique" atom which appears once and only once on each side of the equation and balance it. Do not worry about the rest of the atoms now.
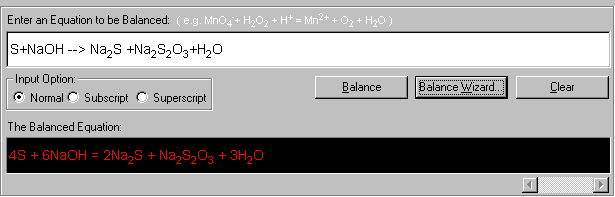
Example I: S + NaOH --> Na2S + Na2S2O3 + H2O
In this case, hydrogen (H) appears in reactant side once in NaOH and in product side once in H2O, we will take care of it first (we explicitly put 1 in front of H2O):
S + 2NaOH --> Na2S + Na2S2O3 + 1H2O
If there are more than one "unique" atoms, balance the one in the compound with more atoms.
Example II: P2I4 + P4 + H2O --> PH4I + H3PO4
Here, although both atoms I and O are "unique" atom, but atom O appears in H3PO4 which has more atoms in total than I containing compounds P2I4 and PH4I. Therefore, we start out with O atom:
P2I4 + P4 + 4H2O --> PH4I + 1H3PO4
4. Click Next to proceed. The second screen appears.
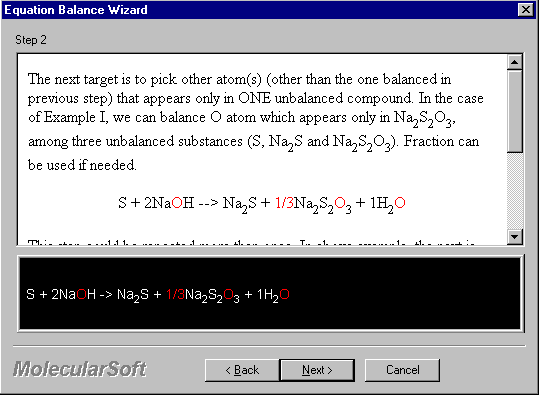
The next target is to pick other atom(s) (other than the one balanced in the previous step) that appears only in ONE unbalanced compound. In the case of Example I, we can balance O atom which appears only in Na2S2O3, among three unbalanced substances (S, Na2S and Na2S2O3). A fraction can be used if needed.
S + 2NaOH --> Na2S + 1/3Na2S2O3 + 1H2O
This step could be repeated more than once.
5. Click Next again. The similar screen appears.
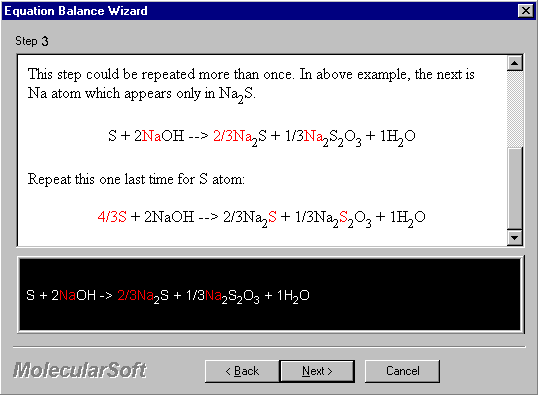
Continue on picking further the unique atom. The next is Na atom which appears only in Na2S.
S + 2NaOH --> 2/3Na2S + 1/3Na2S2O3 + 1H2O
Repeat this one process for S atom:
4/3S + 2NaOH --> 2/3Na2S + 1/3Na2S2O3 + 1H2O
6. Click Next. The final screen of the wizard appears.
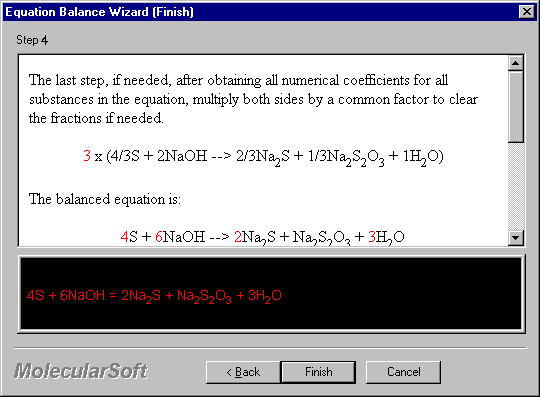
The last step, if needed, after obtaining all numerical coefficients for all substances in the equation, multiply both sides by a common factor to clear the fractions if needed.
3 x (4/3S + 2NaOH --> 2/3Na2S + 1/3Na2S2O3 + 1H2O)
The balanced equation is:
4S + 6NaOH --> 2Na2S + Na2S2O3 + 3H2O
Finally, we have successfully balanced this equation! To review the step-by-step instruction, click on the "<Back" button and then "Next>" button. It is important not only to solve the problem, but also to understand how it is solved. That is a vital aspect of learning - master problem-solving skills, which is what this program intended for.
7. Click Finish button to exit this Balance Wizard and go back to Equation Balance main screen. The balanced equation is now displayed in The Balanced Equation box.