Dilution is the process in which more solvent is added to a solution to lower its concentration. There exists a simple mathematical relationship between the volumes and molarities of the diluted and stock solutions:
Ms x Vs = Md x Vd (1)
which is derived from the fact that the same amount of solute is present in both solutions.
Another way to get the diluted solution is to mix two 'like' solutions, which are solutions contain the same solute and the same solvent. The following formula is used for this case:
(M1 x V1) + (M2 x V2) = Md x Vd (2)
This module is to calculate final solution concentration or other quantities using the above formulae (1) and (2).
Example: What is the molarity of the solution by mixing 60.0 mL of 2.85 M KCl solution with 150.0 mL of 1.10 M KCl Solution?
This is one step process, enter the known data and press Calculate to output the unknowns.
1. Select Dilution link from the front page or Dilution tab from the Solution module. The Input and Output screen appears.
2. In the Input area, enter the four known quantities with a proper significant figure. Select the units associated with the input.
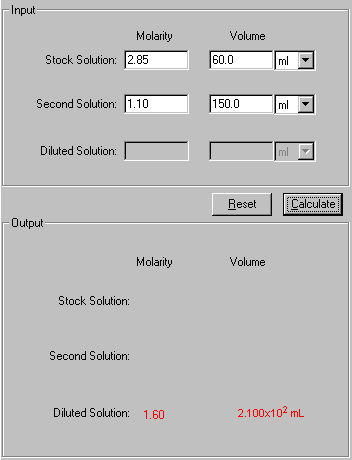
3. Click Calculate to output the answer.
4. The Show Work area on the right shows you step-by-step how your problem has been solved.
To start a new problem, click Reset. All Input fields will be cleared. Follow Step 1-3 again.