Basic Concept
Free Energy, symbolized by G, is a thermodynamic function related to spontaneity. G is defined by the relationship:
G = H - TS
where H is the enthalpy, T is the temperature in Kelvin, and S is the entropy. Therefore, it is especially useful in dealing with the temperature dependence of spontaneity.
Standard Free Energy Change (ΔGº) is the change in free energy that will occur if the reactants in their standard states are converted to the products in their standard states.
One way to calculate ΔGº is to use the equation:
ΔGº = ΔHº - TΔSº
Another way is to take advantages of the fact that free energy is a state function. As calculations in enthalpy changes (ΔHº) and entropy changes (ΔSº):
ΔGºreaction = ΣnpΔGº(products) - ΣnrΔGº(reactants)
Example 1. Calculating ΔGº at 25ºC using ΔHº and ΔSº for the following reaction:
CH4(g) + 2O2(g) = CO2(g) + 2H2O(l)
By the calculations in enthalpy and entropy, the ΔHº and ΔSº of the above reaction is -8.91x105 J and -242 J/K, respectively. The ΔGº can be calculated as:
ΔGº = -8.91x105 J -(298 K) (-242 J/K) = -8.91x105 J + 0.721x105 J = -8.19x105 J = -819 kJ
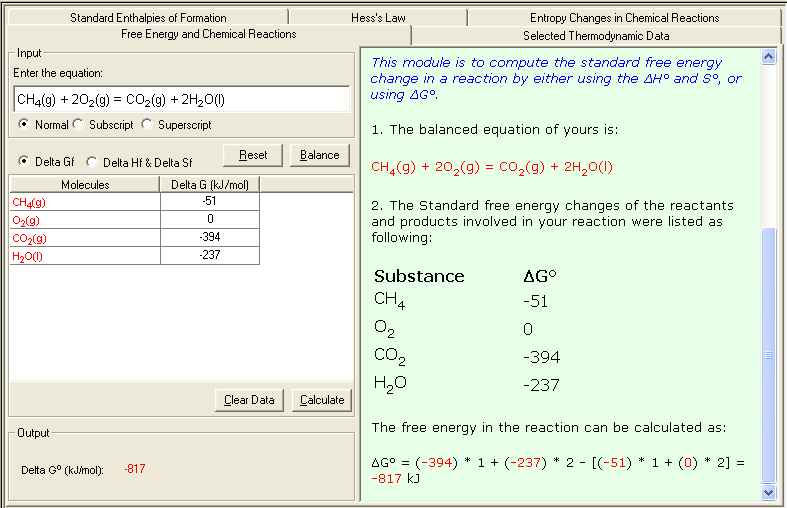
Example 2. Calculating ΔGº using the property of the state function:
Substances ΔGfº (kJ/mol)
CH4(g) -51
O2(g) 0
CO2(g) -394
H2O(l) -237
The standard free energy change of the reaction can be calculated as:
ΔGfº = [(-394) + (-237) * 2] - [(-51) + 0 * 2] = -817 kJ
For the above example,
1. Select Free Energy and Chemical Reactions link either from the front page (Table of Content) or from the Thermochemistry module. The Input and Output screen appears.
2. In the Input area, enter the problem equation; then click Balance button to balance the equation; then enter corresponding Delta G value (ΔG º f ) . You may consult the Selected Thermodynamic Data sub-module for reference data.
3. Click Calculate to output the answer with the detailed step-by-step show work on the side as shown below: