Basic Concept
Entropy (S) is a thermodynamic function, which can be viewed as a measure of randomness or disorder, and describes the number of arrangements (position and/or energy levels) that are available to a system existing in a given state. With entropy, the second law of thermodynamics can be stated "In any spontaneous process there is always an increase in the entropy of the universe." The standard entropy (Sº) of a substance is the value of entropy of the substance at 298 K and 1 atm.
Because entropy is a state function of a system, the entropy change in chemical reactions can be calculated by taking the difference between the standard entropy values of products and those of the reactants:
ΔSºreaction = ΣnpSº(products) - ΣnrSº(reactants)
where the first Σ to take sum of all the products in this reaction, and the second sums all the reactants. The np and nr represent the moles of each product or reactant, respectively.
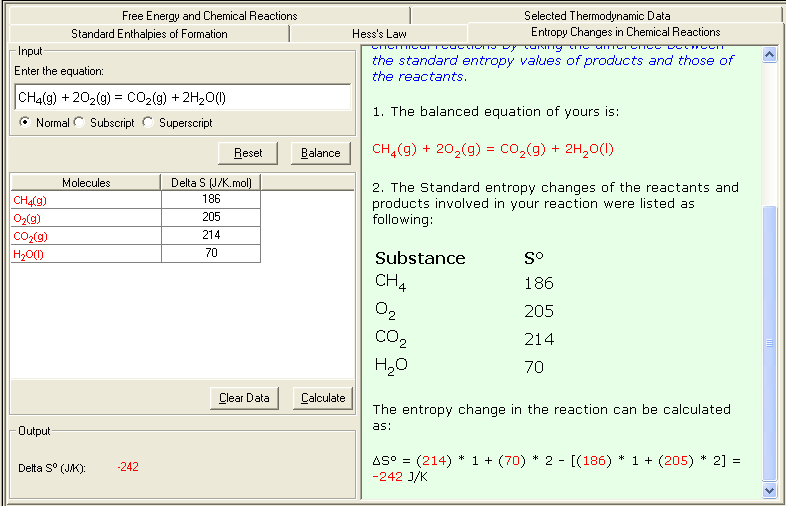
An example calculating the entropy change in a reaction:
CH4(g) + 2O2(g) = CO2(g) + 2H2O(l)
From the Table of Thermodynamics Data, the Standard entropies of the substances involved in above reaction are:
Substances Sº (J/K.mol)
CH4(g) 186
O2(g) 205
CO2(g) 214
H2O(l) 70
The entropy change of the reaction can be calculated as:
ΔSº = [214 + 70 * 2] - [186 + 205 * 2] = -242 J/K
For the above example,
1. Select Entropy Changes in Chemical Reactions link either from the front page (Table of Content) or from the Thermochemistry module. The Input and Output screen appears.
2. In the Input area, enter the problem equation; then click Balance button to balance the equation; then enter corresponding Entropy (S) value. You may consult the Selected Thermodynamic Data sub-module for reference data.
3. Click Calculate to output the answer with the detailed step-by-step show work on the side as shown below: