Basic Concept
Hess's Law is a law of physical chemistry named for Germain Hess's expansion of the Hess Cycle, used to predict the enthalpy change and conservation of energy (denoted as state function ΔH) regardless of the path through which, it is to be determined. The law states that because enthalpy is a state function, the enthalpy change of a reaction is the same regardless of what pathway is taken to achieve the products. In other words, only the start and end states matter to the reaction, not the individual steps between.
Addition of chemical equations can lead to a net equation. If enthalpy change is included for each equation and added, the result will be the enthalpy change for the net equation. This module is to compute the enthalpy change for the net equation by adding the enthalpy change of each included equations.
The Examples utilizing Hess's Law:
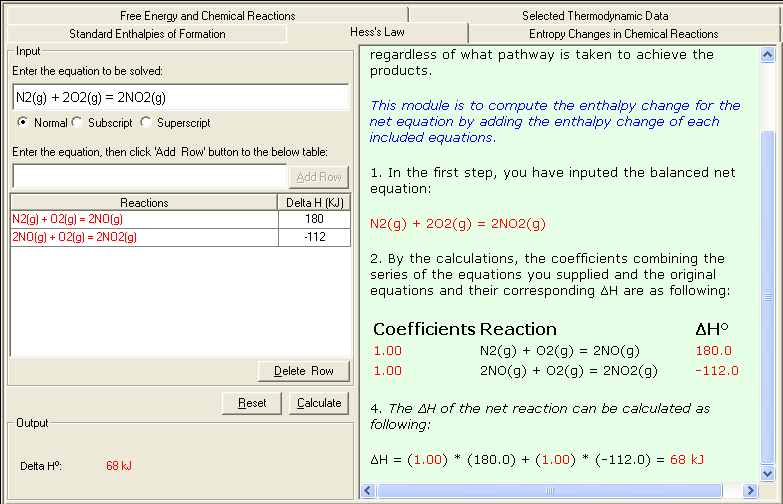
Example 1. The oxidation of nitrogen to produce nitrogen dioxide. The enthalpy change ΔH of the reaction is 68 kJ
N2(g) + 2O2(g) = 2NO2(g) ΔH = 68 kJ
This reaction also can be carried out in two distinct steps:
N2(g) + O2(g) = 2NO(g) ΔH1 = 180 kJ
2NO(g) + O2(g) = 2NO2(g) ΔH2 = -112 kJ
_____________________________________________
Net reaction: N2(g) + 2O2(g) = 2NO2(g)
ΔH = ΔH1 + ΔH2 = 68 kJ
The principle of Hess's law can be shown schematically in the following figure:
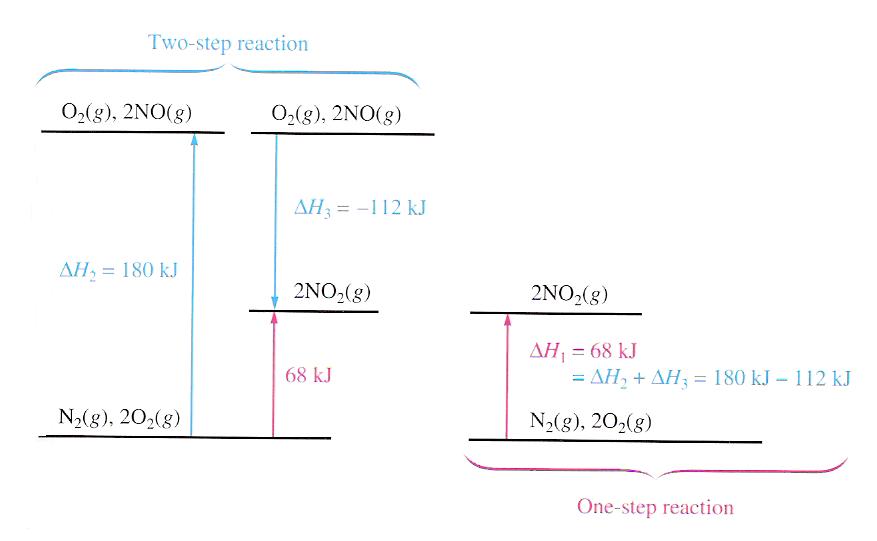
For the above example,
1. Select Hess's Law link either from the front page (Table of Content) or from the Thermochemistry module. The Input and Output screen appears.
2. In the Input area, enter the balanced problem equation; then enter the balanced individual equation to be used; then enter corresponding Delta H value (ΔH f º) . You may consult the Selected Thermodynamic Data sub-module for reference data.
3. Click Calculate to output the answer with the detailed step-by-step show work on the side as shown below: