|
|
A step-by-step solution for YOUR own homework problem!
|
a. For inorganic or some organic components, the program will explain how to draw Lewis Structures step by step
b. Show possible Resonance Structures.
c. Predict Molecular geometries with VSEPR theory.
d. Predict whether a given molecule has a non-zero dipole moment and directional bond polarities based on the electro-negativities of the associated atoms.
e. For diatomic molecules (both homonuclear and heteronuclear), display the MO energy diagrams, while for polyatomic molecules , show the hybrid orbitals
f. Save the step by step Lewis structure results to file
g. Use our pull-down menu of metal cations and nonmetallic anions to form binary compounds and to find out both the formula and name of the compound
Take a Quick Tour (Flash Movies with Audio):(2:25) Broadband | Dial-Up
|
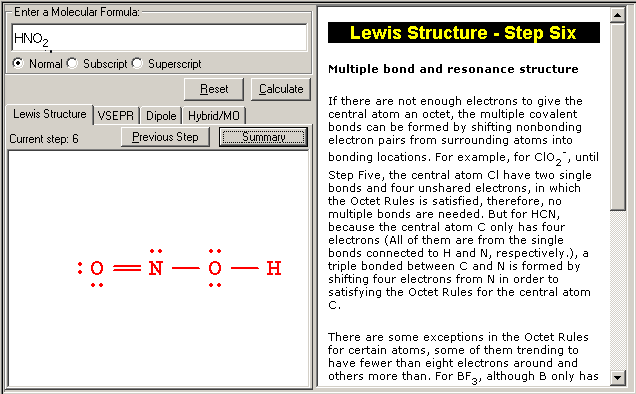
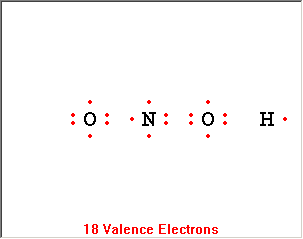
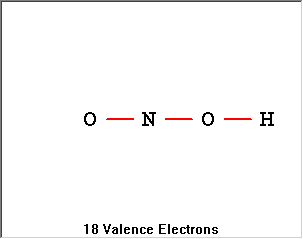
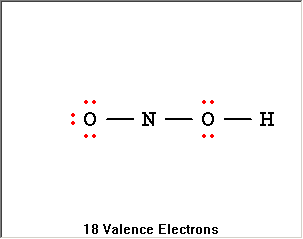
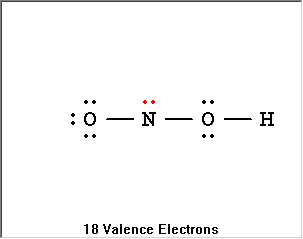
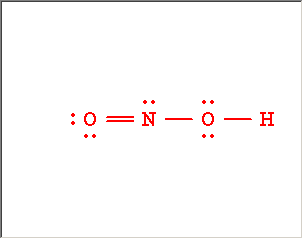
The Lewis Structure Summary |
|
 |
|
 |
|
 |
|
 |
|
 |
|
 |