|
Solve YOUR homework problem with a step-by-step explanation
|
a. Mole calculations
· Calculate molecular weight
· Calculate the percent composition of each element in the molecule
· By entering the formula and any of the three quantities (mass, number of moles, or number of molecules), you can calculate the other two quantities.b. Empirical and Molecular Formulas
· Calculate the empirical formula of a compound by providing the percentage compositions of each element in a molecule, or masses of each element in a sample
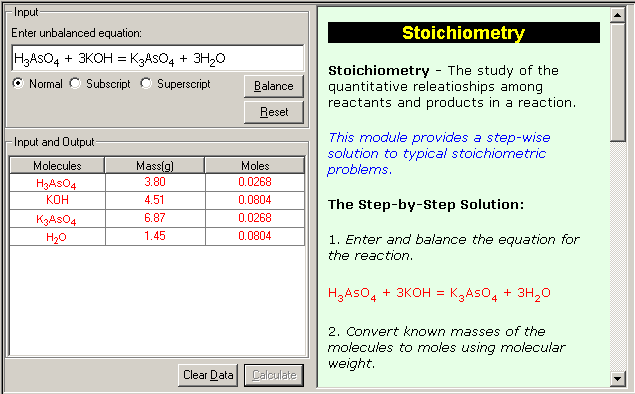
· Calculate the molecular formulas if the molecular weight is knownc. Stoichiometric calculations of any given reaction
· Balance the equation for the reaction
· Can convert masses to moles.
· Convert from moles back to grams using molecular weights.
· Use the balanced equation to set up the appropriate mole ratios
· Use the mole ratios to calculate the number of moles of the desired reactant or product
· Find the limiting reagentd. "Show Work" page displays side-by-side with the solution in explaining how the problem has been solved.